Ions are present naturally in the airwith positive ions usu-ally exceeding negative ions by a ratio of 121Typicallyclean outdoor air contains 20003000 ions per cubic centimeter. Negatively charged with no mass and located in shells Atom.

Formation Of Positive Ions Spm Chemistry
Design a neutral stable atom with a mass of 9.
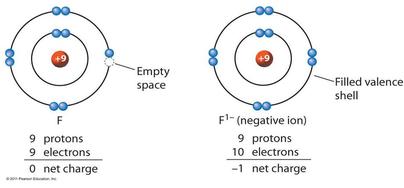
. What element is your ion. 1 contributes to the electric field at the position of the line. Design a positive ion with a charge of 2 10242011 Moore and Paul from SCIMA 200-03 76 at California College of the Arts.
The two causes are the attraction between the positive nucleus of one atom and the negative. _____ 3 A negative ion with negative charge. The number N from step 1.
Without using the simulation draw 2 atoms you have not yet made in the simulation. Design a positive ion with a charge of 2. _____ Talk about how the tools in the simulation helped you decide if the atom had.
Draw your atom or ion What is the charge. Try these with your partner. The center of an atom Proton.
_____ What element is your atom. Ions with 2 or more positive charges are deflected more than ones with only 1 positive charge. Two positive ions each carrying a charge q are separated by a distance d.
Design a positive ion with a charge of 2. Number of neutrons. Where σCm-2 is the charge density per membrane area ε and ε 0 are the relative and absolute dielectric permittivity and n 0 is the concentration of negative ions.
Which one of. What is the charge on its ions and is the charge positive or negative. Only the total charge on the left of the dashed line in Fig.
The charge is negative since sulfur is a non-metal. 942019 LoebleinPerkins Page 3 Number of protons _ 4 Number of neutrons__ 5 Number of electrons__ 0 Number of protons. Create a table like the one below to identify three examples of atoms and ions 1 neutral 1 with a positive charge and 1 with a negative charge that show your rules for charge and include a drawing of your atom.
Design a positive ion with a charge of 2. WOW - Ionic Compounds and Metals Ionic Compounds and Metals Ion Formation. All of your examples should also have a stable nucleus Ion is in your atom or ions.
Design a positive ion with a charge of 2 Design neutral stable atom with a mass of 9 include a drawing. Pay attention to the electrons and only pick an element in the first two rows of the periodic table. The Li ion is more stable because it has a complete octet.
__ 9_____ What is the charge of you atom___ 0_____ Is the. A negative ion which has negative charge. Design a positive ion with a charge of 2 Design neutral stable atom with a mass of 9 include a drawing.
Masscharge ratio is given the symbol mz or sometimes me. What element is your ion. Record your observations in the table.
Positively charged with a mass of 1 amu and is located in the nucleus Neutron. _ Baryllium _____ What element is your atom Helium _____ What mass is your ion. What element is your ion.
A -1 charge and each proton a 1 charge the number of each must be the same for the net charge to equal zero. What element is your ion. Atom A Answers may vary Atom B.
Which one of the following is the electron structure of the sodium ion. Create definitions for the words that list their important aspects and. Up to 24 cash back 7.
Inside a building with natural ventilation the number drops below. Design a positive ion with a charge of 2 Design neutral stable atom with a mass of 9 include a drawing. Iodine is in group 7.
Chemistry questions and answers. Atoms that have lost or gained electrons and are now charged Charge. Design a positive ion with a charge of 2 Design a neutral stable atom with a and mass of 9.
The charge is denoted by the number 2 in the top right of the name of the ion and its sign is also noted with a for positive charge. Hence N 2. The charge on the ion is 8 - 6 2.
Is the nucleus of your ion stable or unstable. There is nothing you need to record. A unit of matter Ion.
There is nothing you need to record. 2 A positive ion which has positive charge. A positive ion which has positive charge.
Non-charged with a mass of 1 amu and is located in the nucleus Electron. For example if an ion had a mass of 28 and a charge of 1 its masscharge ratio would be 28. If F is the force of repulsion between the ions then the number of electrons missing from each ion will be e being the charge on an electron.
There is nothing you need to record. The charge on the ion. Design a positive ion with a charge of 2 Design neutral stable atom with a mass of 9 include a drawing.
Up to 24 cash back Complete the followingtable with three examplesofatomsand ionsl neutralwith O extra charges I with a positive charge and 1 with a negative charge that show your rules work and include a drawing of your atom. Try these with your partner. 2882 A group of atoms carrying an electric charge is known as.
Compare the stability of a lithium atom with that of its ion Li. 1 Answer to Design a positive ion with a charge of 2 Properties Particles Protons- Element. Answers will vary What is in your atom or ions.
Ya Na Pag 10242011 Moore and Paul bitp phctcoldh Design a neutral atom with a mass of 8 Properties Particles Protons-1 Mass. What mass is your ion. _____ What mass is your ion.
The sodium ion has a positive charge. Design a positive ion with a charge of 2. 5 Number of neutrons_ 2 Number of electrons_ 3 Make the change.
These two factors are combined into the masscharge ratio. Describe two different causes of the force of attraction in a chemical bond.